Characterization of Drug Stability
Christin T. Choma
TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA
The shelf life of a pharmaceutical depends ultimately on the stability of the active pharmaceutical ingredient in the formulation. The most common method for characterizing the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions such as high or low pH, high humidity, hydrogen peroxide, etc. The rate of heat evolution by the sample under different stressing conditions provides a direct indication of the stability of the compound under those conditions. Since the measurements are generally completed in a matter of hours, calorimetry provides a rapid approach for screening the stability of compounds; more traditional approaches such as HPLC can then be used to determine the degradation products produced over time.
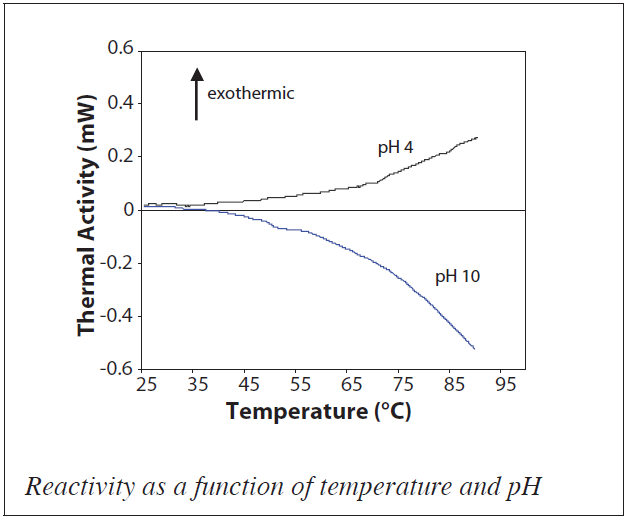
A pharmaceutical compound (10 mg/mL) was dissolved at pH 4 and at pH 10, then both samples were simultaneously heated in a MC-DSC. Thermal activity was evident, indicative of chemical reactions occurring in the samples. Under basic conditions the reaction was endothermic, while in acidic conditions the reaction was exothermic. When the logarithm of the thermal activity was plotted versus the reciprocal temperature, it became apparent that there were at least two reaction pathways with different activation energies at pH 10, while at pH 4 there was only evidence of a single degradative reaction. This information would be very useful in designing traditional HPLC stability screening studies since under basic conditions, this compound should clearly be stressed at lower rather than higher temperatures.