Curing of Epoxy Adhesive Studied by TAM Air
Lars Wadso
BuildingMaterials, Lund University, Sweden
Introduction
Two-componentepoxy adhesives consist of epoxy-resin and a hardener that react to form asolid when they are mixed. The rate of the curing process is very different for different epoxies. Rapid epoxies cure in a few minutes whereas a “standard”epoxy reaches its full strength after more than 24 hours. Isothermalcalorimetry is an excellent technique to follow such a process.
Materials and method
An experiment was performed where a large batch of epoxy adhesive (Araldit Professional, Ciba, Switzerland) was mixed according to the manufacturers’guidelines. Eight samples of 7.1 -11.2 g were loaded into glass ampoules.Hardening was then recorded for 45 hours at 22oC.
Results and discussion
Figure1 shows the heat production rate as a function of time. It can be seen that the spread of results is low and that after an initial reaction period of fivehours the heat production rate decrease is similar to an exponential decay.
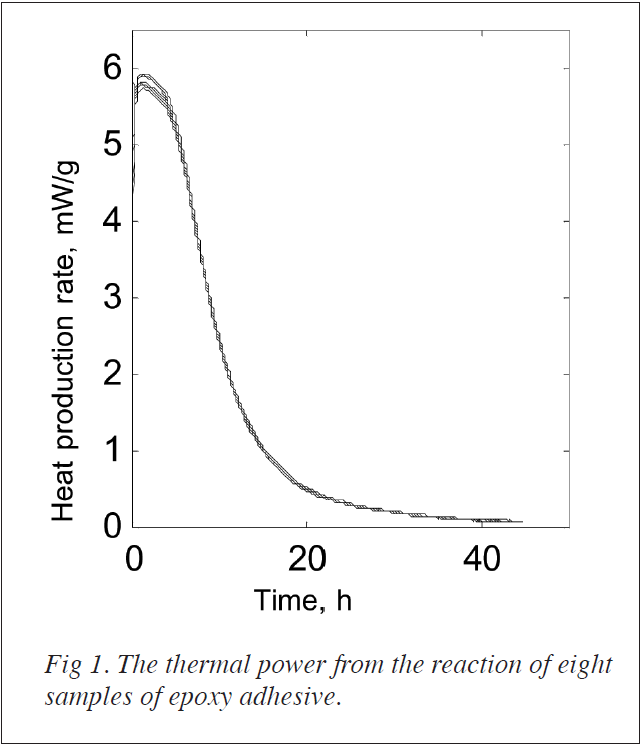
After 45 h the thermal power is approx. 0.06 mW/g, i.e. 600 μW for a l0g sample. As the detection limit for TAM Air is better than 3 μW it would stillbe possible to follow the reaction for an even longer time than was done here.
In figure 2, the same data as in figure 1 is plotted with logarithmic axes. The logarithmic time starts at 1 hour and is extended to 100 hours.
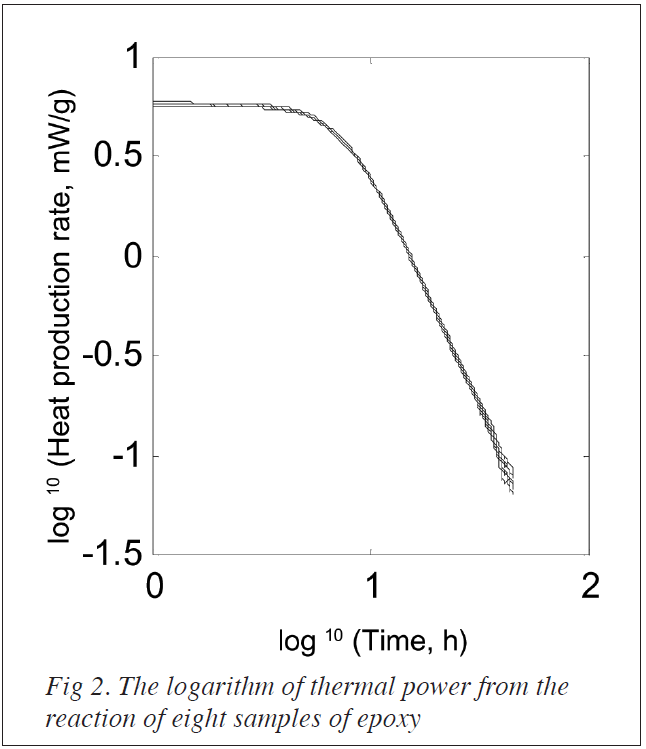
An interesting application of this type of measurement is the assessment of the fraction of unhardened epoxy as a function of time. However, to do this a modelfor the hardening process is required. Such a model should describe the completehardening process, e.g. movement and diffusion in the stiffening polymernetwork. To show the principle we assume that the linear rate dependence seenin the logarithmic scales in figure 2 between 10 and 45 hours is also validafter 45 hours. It is thus possible to extrapolate the rate development intothe future.
The heat production rate is proportional to the rate of the reaction and the totalheat produced is proportional to the extent of the reaction. This may be usedto test reaction models and calculate the parameters of such models [1]. The totalheat produced during the 45 hour experiment was approx. 600 J/g. If we assumethat most of the reaction has taken place at this time and that we have thesame chemical reaction during the whole hardening process, we may calculate therelative rate of the reaction at any time. The initial and final heat productionrates measured were 6 and 0.06 mW/g. Dividing by a total heat of 600 J/g givesrelative reaction rates of 4 and 0.04 percent reaction per hour at the startand end of the measurement. The measurements described above were made with TAMAir. However, for the study of the hardening process over a longer time themore sensitive microcalorimeter 2277 TAM is recommended.
Further experiments
Another interesting application of this instrument is to study the influence of other materials and substances on the hardening process. For example, different types of surfaces or contaminants with which an epoxy adhesive might come into contactcan be studied. It is also possible to assess the influence of other factorssuch as temperature, mixing ratios, mixing intensity etc. on the hardeningprocess.
Reference
Willson R.J., Beezer, A. E., Mitchell. J.C., and Loh, W. (1995) “Determination of thermodynamicand kinetic parameters from isothermal heat conduction microcalorimetry: applicationsto long-tern-reaction studies” J. Phys. Chem. 99 7108-7113

