
期刊:ACS Appl Mater Interfaces
主题:可降解生物材料周边微环境pH空间分布及其对破骨细胞活性的调节作用
标题:Spatial Distribution of Biomaterial Microenvironment pH and Its Modulatory Effect on Osteoclasts at Early Stage of Bone Defect Regeneration
影响因子:8.097
监测指标:H+流速
检测部位:生物材料玻璃材料
H+流速流实验处理方法:
不同配比的玻璃材料
H+流速流实验测试液成份:
模拟体液
作者:中科院深圳先进技术研究院潘浩波、刘文龙
文献简介
2019年2月,中科院深圳先进技术研究院潘浩波研究员团队发表了题为 “Spatial Distribution of Biomaterial Microenvironment pH and Its Modulatory Effect on Osteoclasts at the Early Stage of Bone Defect Regeneration” 的文章,通过关联可降解生物材料周边微环境H+离子分布和破骨细胞活性,旨在描绘 “材料-机体” 交互作用之 “微环境” 分布范围。相关成果发表于ACS Applied Materials and Interfaces。
近年来,由人工组织与机体微环境交互作用而引起的材料学及生物学效应受到越来越多的关注。团队早期研究发现pH值在调控骨修复过程中破骨与成骨间平衡具有重要作用,并采用微电极技术初步探明存在微碱性范围内的某一pH 阈值,使得成骨细胞、破骨细胞及骨髓基质干细胞在阈值两侧的活性产生明显变化(即“开/关”效应)(Liu WL et al., Osteoporosis International, 2016; Shen YH et al., Journal of Materials Chemistry, 2012; Shen YH et al., Langmuir, 2011)。进而,本研究采用非损伤微测技术,考察了系列可降解材料近表面(50-3000μm)氢离子流速及空间分布的梯度效应。相对于表面pH微电极,NMT系统在检测信号种类,空间和时间的可控性等方面展现出了独到的优势。
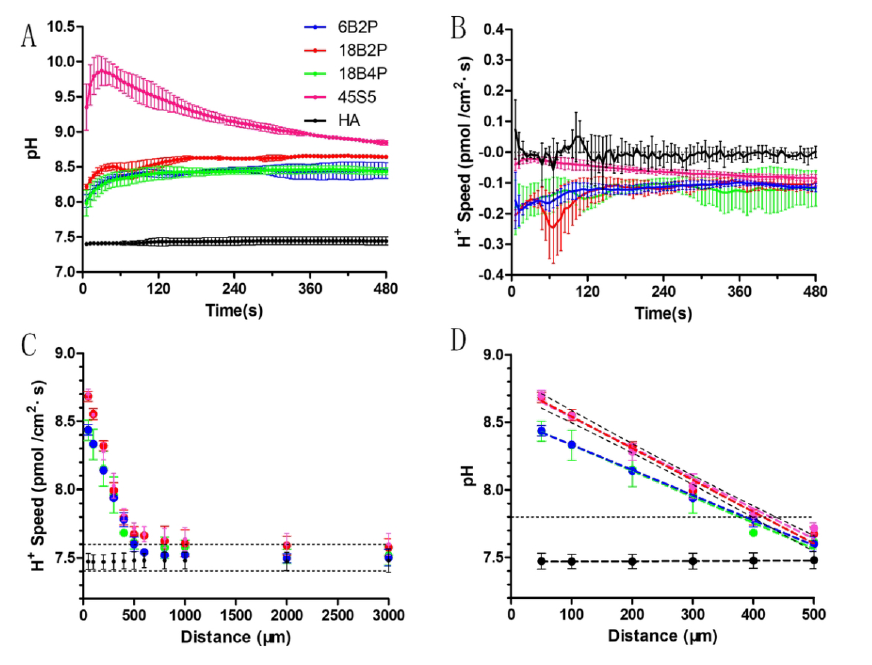
结果证明,破骨细胞在微碱性环境中(pH>7.8)的分化及侵蚀骨板能力基本丧失;基于此,团队制备了系列碱性可降解硅硼酸盐玻璃,并使用NMT系统描绘出与破骨细胞产生“开/关”效应相对应的材料表面微环境的影响范围(400 ± 50 μm)。为研究“材料-骨组织”早期的相互作用,团队使用最新建立的小鼠骨缺损动物模型(Liu WL et al., Tissue Engineering Part C: Methods, 2016),证明了材料周边碱性微环境能促进骨质疏松骨缺损的快速再生。本研究进而表明,对微环境离子浓度的精确调控将为未来新材料的设计提供指导意义。
英文摘要
It is generally accepted that biodegradable materials greatly influence the nearby microenvironment where cells reside; however, the range of interfacial properties has seldom been discussed due to technical bottlenecks.
This study aims to depict biomaterial microenvironment boundaries by correlating interfacial H+ distribution with surrounding cell behaviors. Using a disuse-related osteoporotic mouse model, we confirmed that the abnormal activated osteoclasts could be suppressed under relatively alkaline conditions. The differentiation and apatite-resorption capability of osteoclasts were “switched off” when cultured in titrated material extracts with pH values higher than 7.8.
To generate a localized alkaline microenvironment, a series of borosilicates were fabricated and their interfacial H+ distributions were monitored spatiotemporally by employing noninvasive microtest technology. By correlating interfacial H+ distribution with osteoclast “switch on/off” behavior, the microenvironment boundary of the tested material was found to be 400 ± 50 μm, which is broader than the generally accepted value, 300 μm.
Furthermore, osteoporotic mice implanted with materials with higher interfacial pH values and boarder effective ranges had lower osteoclast activities and a thicker new bone.
To conclude, effective proton microenvironment boundaries of degradable biomaterials were depicted and a weak alkaline microenvironment was shown to promote regeneration of osteoporotic bones possibly by suppressing abnormal activated osteoclasts.
中文摘要(谷歌机翻)
人们普遍认为,可生物降解的材料会极大地影响细胞所在的附近微环境。然而,由于技术瓶颈,很少讨论界面性质的范围。
这项研究旨在通过将界面H+分布与周围细胞行为相关联来描绘生物材料微环境的边界。使用与废品相关的骨质疏松小鼠模型,我们确认了在相对碱性条件下可以抑制异常活化的破骨细胞。当在pH值高于7.8的滴定材料提取物中培养时,破骨细胞的分化和磷灰石吸收能力被“关闭”。
为了产生局部的碱性微环境,制造了一系列的硼硅酸盐,并采用无创微测试技术对它们的界面H+分布进行了时空监测。通过将界面H+分布与破骨细胞的“开/关”行为相关联,发现被测材料的微环境边界为400±50μm,比公认的值300μm宽。
此外,植入具有较高界面pH值和边界有效范围的材料的骨质疏松小鼠的破骨细胞活性较低,新骨较厚。
总而言之,描绘了可降解生物材料的有效质子微环境边界,并显示了弱碱性微环境可能通过抑制异常活化的破骨细胞来促进骨质疏松骨骼的再生。
