
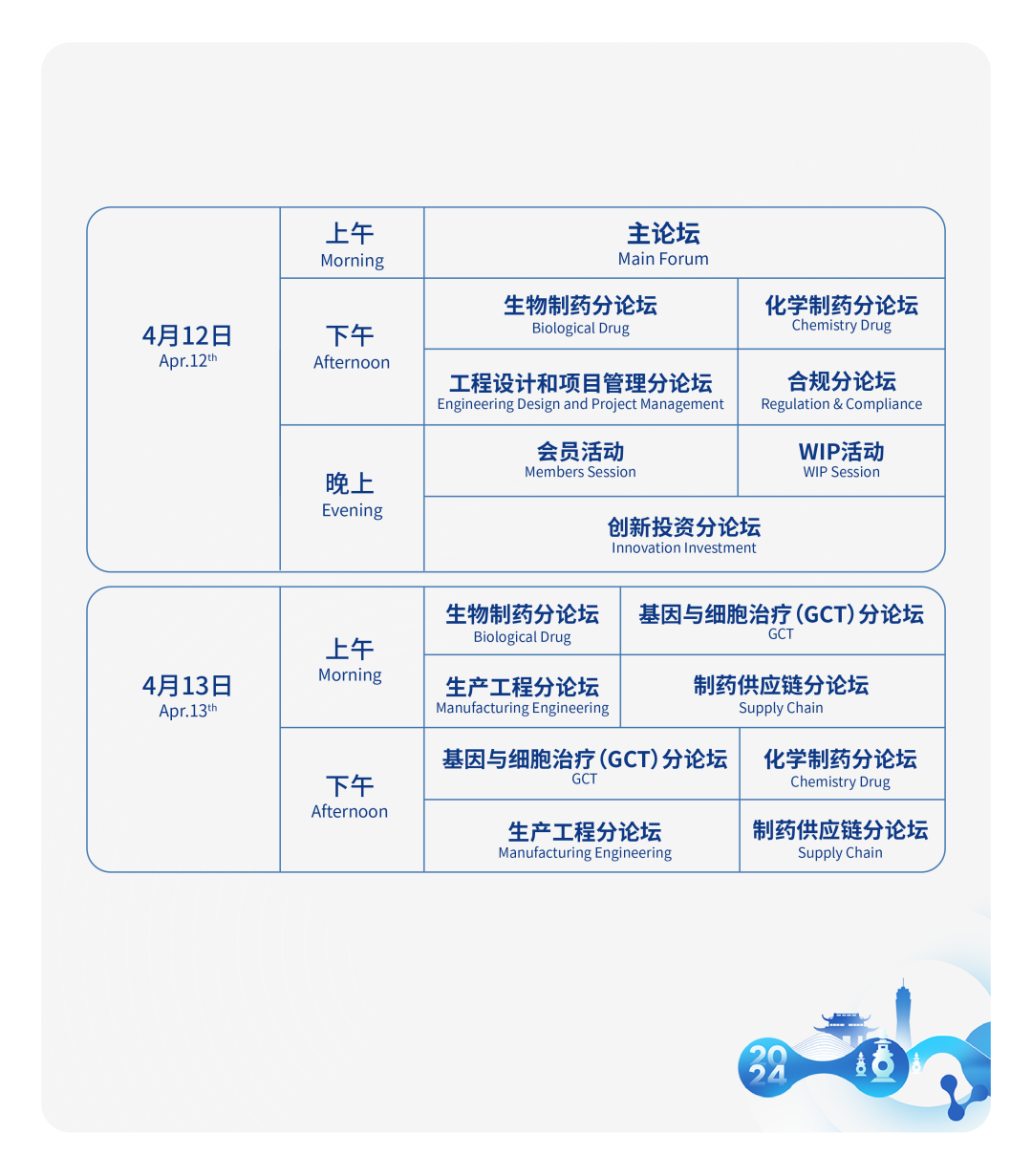
9个论坛
66个学术主题,13位国际讲者
现场近500余人参会,线上超万人观看
会议时间、地点
2024年4月12~13日
(周五~周六)
中国·杭州
浙商开元名都酒店
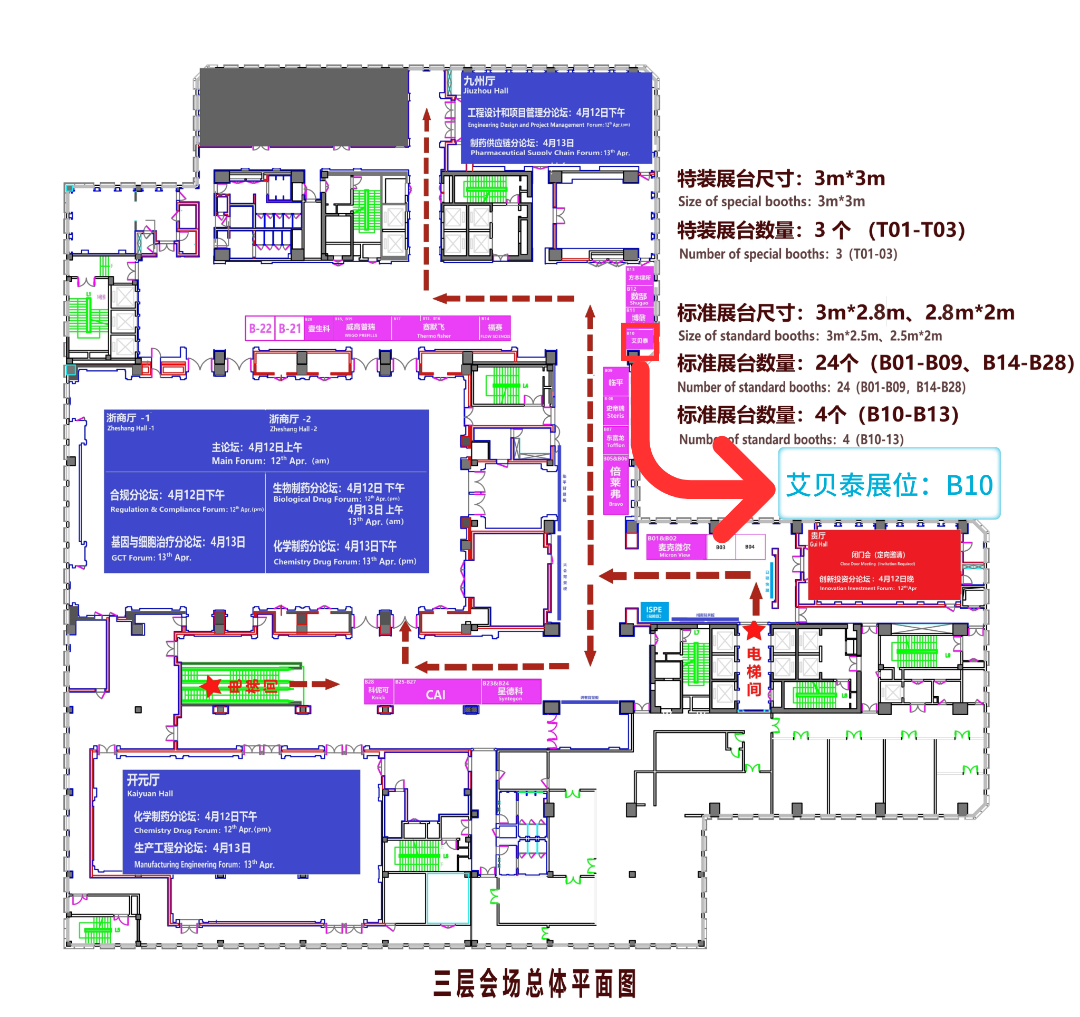
艾贝泰展位号:B10
会议日程

主论坛
Main Forum
4月12日 12th Apr.(周五Fri.)
08:30-12:00
主持人暖场致辞
Warmup Speech
大会开幕启动仪式
Opening Ceremony
FDA中国办公室官员演讲
FDA China Official Speech
FDA‘s view point on advanced pharmaceuticals manufacturing

贝妮娜 Tonia Bernard
公共卫生硕士
助理主任,FDA中国办公室
MPH
Assistant Country Director, FDA China Office
FOYA 2023获奖介绍
FOYA 2023 Introduction
八大论坛,聚焦制药工程全链路
包括生物制药、基因与细胞治疗(GCT)、化学制药、工程设计和项目管理、生产工程、合规、制药供应链、创新投资八大论坛版块,涉及到行业内各个领域的动态。
生物制药分论坛
Biological Drug Forum

李锦才 Jimmy LI
药明合联 CEO
CEO,WuXi XDC
聚焦生物制药行业高度关注话题,结合指南,分享企业实操经验
Focusing on the hot topics of the Biologics industry, sharing relevant guidance documents and good practices from pharmaceutical companies.
4月12日 12th Apr.(周五Fri.)
13:00-16:30
隔离器无菌生产的挑战
Challenges with Aseptic Production in An Isolator

顾晨 Chen GU
技术总监,星德科包装技术(杭州)有限公司
Technical director,Syntegon
指南介绍:活性化合物的遏制
Introduction for Good Practice Guide: Containment for Potent Compound

Dr. Rainer Nicolai
Product Owner Engineering Consulting
F. Hoffmann - La Roche AG
高活化合物的处理和操作
Handling and Operation of potent compounds

朱人 William Ren ZHU
Golder IH业务负责人,科进公司
Associate Director, Environment, WSP
企业对高活化合物的综合管理
Management of highly potent compounds by pharmaceutical companies

罗建军博士 Dr. J.J LUO
生物偶联产品研发和生产副总裁
药明合联
Vice President of Bioconjugate Product Development and Manufacturing WuXi XDC
4月13日 13th Apr.(周六 Sat.)
09:00-12:00
指南介绍:APQ:PPPQMS
Introduction for APQ Guide:Process Performance & Product Quality Monitoring System (PPPQMS)

Mr. Maurice B. Parlane B Tech MIT
Principal
New Wayz Consulting Ltd
持续工艺监控和改进
Continued process monitoring and improvement

温源博士 Dr. Yuan WEN
全球生产部MFG1和MFG4的负责人,药明生物
head of MFG1 and MFG4 of Global Manufacturing,WuXi Biologics
自我注射系统及新材料预灌封系统在注射剂上的应用
Application of self-injection system and polymer materials in injections

郭融 William GUO
研发副总裁
上海新耀湃科医疗科技股份有限公司
Vice President R&D for Shanghai Innopac
国产生物创新药出海美国策略分享

王刚 Gang WANG
执行董事、高级副总裁兼首席质量官
上海君实生物工程有限公司
Executive Director, Senior Vice President and Chief Quality Officer
Shanghai Junshi Biotechnology Co., Ltd
新型一次性碟片式离心机在收获工艺环节的应用
Maximizing Efficiency and Cost-saving:Thermo Fisher Single-use Centrifuge for harvest process

张龙浩 Longhao ZHANG
生物工艺部技术支持经理,赛默飞世尔科技
Technical Support Manager, Bioprocess Department,Thermofisher
国内企业接受EMA PAI检查的经验分享
Experience sharing of China-based pharma company preparing for EMA Pre-Approval Inspection (PAI)

黄玮 Wei HUANG
总裁,上海复宏汉霖生物制药有限公司
President
Shanghai Henlius Biopharmaceutical Co.,Ltd.
生产工程分论坛
Manufacturing Engineering Forum

甘益民 Yimin GAN
上海复旦张江生物医药股份有限公司副总经理
Vice General Manager,Shanghai Fudan Zhangjiang bio-pharmaceutical Co.,Ltd.
聚焦指南有关清洁验证和无菌生产设施,为制药业答疑解惑
Focus on latest guidance about cleaning validation and sterile manufactiring facilities, aims to offer an instructive solution for Pharma Manufacturer.
4月13日 13th Apr.(周六 Sat.)
09:00-12:10
清洁验证指南解读
Guide introduction: Cleaning Validation Lifecycle - Applications, Methods, & Controls

Ms. Catherine T. Oakes
Managing Director
Oakes Group Global Ltd.
标准化和自动化对于提高产品合规性与生产效率的作用与意义
How standardization and automation improve compliance and productivity

Martin W. Goebel
Sales Director Analytics
Knick GmbH, Germany

马强 QIANG MA
Sales Manager - Analytics
Knick (Shanghai) Electronic Measurement Trading Co., Ltd
清洁验证中条件选择与评估
Worst case assessment in cleaning validation

牛萍 Ping NIU
质量与合规高级顾问,齐鲁制药
Sr. Consultant of Quality and Compliance,Qilu Pharmaceutical
生物制药清洁验证经验分享
Biotechnology cleaning validation good practice sharing

Richard Chai
Senior Technical Service Manager
STERIS Corporation
清洁验证在中药生产中的实施
Cleaning validation in Chinese medicine manufacturing

孙英强 Yingqiang SUN
质量经理,步长药业
Quality Manager,Buchang Pharmaceutical
13:30-17:30
Operational Readiness

Mr John Vaughn
Vice President, Asia,CAI
新版欧盟GMP无菌附录的主要变化及无菌保障能力提升策略
Major Changes in EU GMP Annex 1 Manufacture of Sterile Medicinal Products and Strategies for Enhancing Sterile Assurance Capabilities

张新 Xin ZHANG
顾问教授,沈阳药科大学
Consultant professor
Shenyang Pharmaceutical University
无菌药品生产设施指南解读
Baseline Guide Vol Vol 3 introduction: Sterile Product Manufacturing Facilities 3rd Edition

Mr.Aaron Weinstein
Senior Director,Compliance Consulting
IPS-Integrated Project Services,LLC
欧美法规中关于清洁消毒的若干要点解读
Lessons learnt on cleaning & disinfection regulatory observations
刘寅 Yin LIU
生命科学中国区技术支持,STERIS
隔离器的灭菌与验证
Sterilization and validation for Isolator

柯桂盛 Guisheng KE
海普瑞
Shenzhen Hepalink Pharmacetical GroupCo.,LTD
问与答
Q&A
合规分论坛
Regulation & Compliance Forum

孙京林 Jinglin SUN
中国生物技术股份有限公司副总裁
VP,China National Biotec Group(CNBG)
聚焦制药行业形势变化,关注生物制品MAH制度、质量风险管理等热点问题
Focus on the latest change of pharmaceutical industry, pay attention to hot topics such as the implementation of the MAH system for biological products and the quality risk management.
4月12日 12th Apr.(周五 Fri.)
13:00-17:40
生物制品MAH制度
implementation of the MAH system for biological products

潘海龙 Hailong PAN
质量管理部主任,中国生物技术股份有限公司
Director, Quliaty Managemetn Dept. CNBG
浅析无菌操作的发展趋势
operation trend in aseptic production

娄再飞 Zaifei LOU
验证与合规部门负责人
星德科包装技术(杭州)有限公司
与国际接轨-中国版《无菌药品生产污染控制策略(CCS)技术指南》介绍

肖志坚 Zhijian XIAO
中国质量负责人,百济神州
主任,中国医药设备工程协会无菌药品先进制造专业委员会
Head of Quality in China, Beigene
Director, Professional Commitee on Advanced Manufacturing of aseptic drugs, CPAPE
国际典型无菌检查缺陷分析及对中国企业的启示
Typical observations analysis in international aseptic inspections and the enlightenment for Chinese enterprises

韩亮 博士 Dr. Liang HAN
研究顾问,北京大学知识工程与监管科学实验室,识林知识平台负责人
Research Consultant, Knowledge Engineering and Regulatory Science Laboratories, BeijingUniversityHead of Shlinx
Panel
孙京林、潘海龙、娄再飞、肖志坚、韩亮、MHRA专家
Jinglin SUN, Hailong PAN,Zaifei LOU, Zhijian XIAO, Liang HAN,MHRA SME
工程设计和项目管理分论坛
Engineering Design and Project Mgt Forum

康 伟 Wei KANG
香港奥星集团副总裁
VP,Austar Group
聚焦于生物制药设施GMP合规设计与项目管理,依托良好实践指南:良好工程实践(2021)的指导,深度解析并分享企业在这方面的实战经验
Focusing on GMP compliance design and project management of biopharmaceutical facilities, we will delve into the interpretation of Good Practice Guide: Good Engineering Practice (2021) and share practical experiences from industry operations.
4月12日 12th Apr.(周五 Fri.)
13:00-16:15
GEP指南介绍
GEP Guideline Introduction

Chip Bennett
良好实践指南:良好工程实践,第二版(2021年) 主要作者
Associate Director, Global C&Q | CAI
Lead Author on the Good Practice Guide: Good Engineering Practice, 2nd Edition (2021)
欧盟标准的无菌制剂厂房的污染控制策略
——基于欧盟新版附录1案例分享
Eu standard contamination control strategy for aseptic filling plant -- Case sharing based on the new EU Appendix 1
企业解决方案高质量、低风险,快速灵活的赋能您的生物制药产能建设
Enterprise Solutions enabling your biomanufacturing capacity with high quality, low risk and high speed and flexibility

何永江 Yongjiang HE
企业解决方案高级项目经理,Cytiva
Senior Project Manager, Enterprise Solutions, Cytiva
生物制药厂房设计与实施:策略优化与案例分析
Design and Implementation of Biopharmaceutical Manufacturing Facilities: Strategy Optimization and Case Study

Mark Stephens
Global Subject Matter Expert,Exyte
血液制品生物工厂项目建设管理要点
Key points in the construction and management of plasma manufacturing projects

郭维强 Weiqiang GUO
上海血制副总经理,天坛生物
Vice General Manager, Tiantan Bio
化学制药分论坛
Chemistry Drug Forum

王卫兵 Alex WANG
江苏恩华药业股份有限公司副总裁
VP,Jiangsu Nhwa Group
2023年3月6日,国家药监局核查中心(CFDI)组织研究起草的《药品共线生产质量管理指南》正式稿发布。国际上,ICH Q9 R1《质量风险管理》修订版定稿的发布,结合欧盟早已颁布的EU GMP附录1《无菌产品生产》,制药行业对于生产的各个环节以及产品整个生命周期里的风险管控越来越重视,尤其是污染和交叉污染的防控。国际制药工程协会针对药品生产风险管理,高活性产品共线生产等各个方面也有专门的指南,欢迎行业同仁一起来分享探讨,合作共通
In March 6th, 2023, CFDI from NMPA published the official version of a guidance on Drug Manufacture with Shared Facilities. World widely, ICH Q9 “Risk Management” R1 and EU GMP Annex 1 remain hot topics of the industry, which is embraced cross the product lifecycle, especially risk of contamination & cross contamination once again becomes critical for pharmaceutical operations. Along with it, established series guidelines on those topics and we are going to discuss it during the conference. Your participation and sharing are welcome.
4月12日 12th Apr.(周五 Fri.)
13:00-17:20
基于CCS的环境监控解决方案
CCS-based Environmental Monitoring Solution

徐敏凤 Minfeng XU
产品总监,麦克微尔(上海)科技有限公司
Chief Product Officer,Micron view
CCS :污 染 控 制 策 略/附 件 1
CCS: Contamination Control Strategy/Annex 1

徐禾丰 Hefeng XU
行业专业人士 Industry professional
新版欧盟附录1的主要变化及湿热灭菌方面的实践解读
key highlight of revised Annex l of EU GMP and example sharing in moist heat sterilization

胡仲新 Jessica Hu
资深质量总监,百特上海医疗仪器有限公司
Sr. Director, Quality ,Baxter Healthcare
指南:基于风险的药品生产第2版
Guidance: Risk-Based Manufacture of Pharma Products 2nd Editio
Stephanie A. Wilkins,PE
PharmaConsult US, Inc
基于风险的生产——理论与实践
Risk-based production - Theory and practice
张新 Xin ZHANG
顾问教授,沈阳药科大学
Consultant professor
Shenyang Pharmaceutical University
4月13日 13th Apr.(周六 Sat.)
13:30-17:30
运营管理应用分享
API: Good Practice Guide: Operations Management (guideline), intergrate with ICH Q11

周臻弘 Zhenhong ZHOU
质量副总裁,浙江海正药业
VP Quality,Zhejiang Hisun Pharmaceutical Co., Ltd
良好规范指南:有效化合物的防护+共用设施
Good Practice Guide:Containment for Potent Compounds+ Shared facilities

夏禄华 Luhua XIA
副总经理,江苏金迪克生物科技有限公司
生命周期管理:APQ指南:过程性能和产品质量监控系统(PPPQMS) (指南)
Lifecycle Management : APQ Guide: Process Performance & Product Quality Monitori ng System (PPPQMS) (guideline)
Mr. Maurice B. Parlane, B Tech MIT
Principal
New Wayz Consulting Ltd
PPPQMS及其在固体制剂的应用
PPPQMS and its application in solid preparations

陈四向 Sixiang CHEN
广东东阳光药业股份有限公司 专家
Guangdong Sunshine Lake Pharm Co.,Ltd Expert
基因与细胞治疗(GCT)分论坛
GCT Forum

高 杨 Frank GAO
行业专家
基于经典制药实践,赋能新兴疗法发展
Leverage classic pharmaceutical best practice to empower the development of advanced theraputic medical products.
4月13日 13th Apr.(周六 Sat.)
09:00-12:10
“先进治疗产品-自体细胞治疗指南”指导原则讲解
Introduction for Guide:ATMPs - Autologous Cell Therapy

Jeff Odum
Practice Leader:ATMPs & Biologics
Genesis AEC
CGT设施如何设计和进行风险控制-基于指南&GMP规范
How to design GMP facilities and conduct risk control - based on guidelines &GMP specifications

付长亮 Changliang FU
SME,奥星集团
从毒菌污染看微生物控制策略
Looking at microbial control strategies from the perspective of moldcontamination

王晓明 Xiaoming WANG
首席顾问,瓦格纳生物科技咨询
制药企业的供应链体系
A world-class supply chain for pharmaceutical companies

丛征宇 Zhengyu CONG
高级副总裁,驯鹿生物
13:00-16:50
CGT产品GMP审计中发现的问题与分析
Analysis of defects found in GMP audits of CGT products

董立玮 Liwei DONG
亚太区首席合规顾问,精鼎医药
Principal Consultant APAC Compliance,Parexel International
细胞治疗/溶瘤病毒/AAV产品药学变更的CMC考虑
CMC considerations for pharmaceutical changes in cell therapy/oncolytic virus /AAV products

曹晓平Ph.D. Xiaoping CAO Ph.D
CTO,药明巨诺
CTO,JW THERAPEUTICS
对FDA关于细胞基因治疗产品Potency方法建立指南的考虑
Consideration of FDA guidelines for the establishment of bioassay methods for CGT

刘雅容Ph.D Yarong LIU Ph.D
CEO,沙砾生物
CEO,GRIT Biotechnology
细胞治疗上市前工艺验证
Cell therapy pre-NDA process validation

王永增 James WANG
CTO, 合源生物
CTO, JUVENTAS Bio
针对细胞和基因治疗的脱靶和随机插入的分析
Analysis of off-target and random insertions for cell and gene therapy

谭炳合,Ph.D,
生产与CMC副总裁,邦耀生物
Vice President, BRL Medicine
制药供应链论坛
Pharmaceutical Supply Chain Forum

王 麟 Lynn WANG
Executive Director, Global Project Management, Wuxi XDC
聚焦医药工程供应链的挑战和热点,结合生产供应链,临床供应链和供应链数字化方面的更新和指南,探讨中国出发的全球医药工程供应链实践
Focusing on the challenges and hot topic of the pharmaceutical engineering supply chain, to explore the best practices of the global pharmaceutical engineering supply chain from China to global on manufacturing, clinical supply chain and supply chain digitization, with the market updates sharing and guidelines discussion.
4月13日 13th Apr.(周六 Sat.)
09:00-12:10
数字系统_临床供应链的期望和价值主张
Digital Systems_Expectations and Value Proposition for Clinical Supply Chain

Glasser, Barrett MBA, MPH
Senior Director, Global Clinical Supply Chain,Team Leader of Digital Clinical Supply Chain and Process Excellence
Takeda
美国食品药品监督管理局的检查计划:基于风险的设施评估方法
US FDA's Inspection Program: A Risk Based Approach to Facility Assessments

Dr. Zhihao Peter Qiu
External Advocacy Lead APAC
Roche Genentech
ICH Q5A 法规更新后对生物安全检测的未来展望
Implementation of lCH Q5A: Expectations and Recommendations for Biosafety Testing for Biosafety of Medicinal Products

吴云飞 Dr. Yunfei Wu
技术与法规经理,Merck
Technical and regulatory Manager,Merck
AI赋能医药智造的实践
The latest practices of Al in smart manufacturing

陶静雯 Tracy TAO
量子创新场主任
明度智云
Director of AI innovation department
Mingdu Zhiyun Technology Co.,Ltd
13:00-16:25
利用供应商质量管理系统和全球化布局保障药品的生产安全
Utilizing a supplier quality management system and a globalized layout to ensure the production safety of pharmaceutical products

林森 Nathan Lin
上游工艺解决方案技术总监,默克
多国家多中心临床试验中的质量管理策略
Quality Management Strategy in Multiple Regions Clinical Trials (MRCT)

施维维 Vela Shi
临床试验事业部中国质量负责人
赛默飞
Head of Quality, Thermo Fisher Clinical Trail Division China
临床试验供应链的仿真及关键决策优化
Clinical Supplies Simulation and Strategic Decision Optimization

付迪宇 Diyu FU
创始人,战略及业务发展负责人,华升智药科技开发(北京)有限公司

茅亚超 Yachao MAO
联合创始人,产品研发负责人,华升智药科技开发(北京)有限公司
临床供应质量管理策略
Quality Management Strategy for Clinical Supply

高晓伟 Daniel Gao
副总裁,广州汉腾生物科技有限公司
CGT的全球临床供应实践
Global Clinical Supply Practices for CGT
韦庆坤 Qingkun WEI
高腾生物
创新与投资分论坛
定向邀请(Invitation Reqired)
Innovation Investment Forum

庞飞飞 Sophie PANG
董秘,北京鼎持
Board Secretary,Beijing Dingchi Co.
本论坛旨在充分利用平台资源优势,建立机制、凝聚共识、形成合力,围绕专业+产业链构建协作支撑的资金链,携手共营良好创投氛围,共搭投融资合作平台,共促协同创新、成果转化和产业协作
This forum aims to make full use of the resource advantages of platform, establish mechanisms, build consensus, form synergy, build a collaborative supporting capital chain around the professional + industrial chain, work together to build a good venture capital atmosphere, build investment and financing cooperation platforms, and promote collaborative innovation, achievement transformation and industrial collaboration.
4月12日 12th Apr.(周五 Fri.)
17:30 - 19:10
投行角度分享
Care Sharing on Investment Bank Perspecitve

吴虹生 Hongsheng WU
执行董事,招商证券投资银行委员会
Executive director, China Merchants Securities and Investment Banking Commission
国有资本视角
融资成功案例
Best Case sharing of Successful Financing

庞飞飞 Sophie PANG
董秘,北京鼎持
Board Secretary,Beijing Dingchi Co.
中国医药企业赴美投资动因观察
Observation on the motivation of Chinese pharmaceutical enterprises to invest in the United States

胡晓蕾 Rosemary Hu
合伙人,税务及商务咨询,企业并购重组税务服务,德勤中国
Partner,Tax& Business Advisory ServicesMergers & Acquisitions
Deloitte China
马里兰州助力中国药企出海美国市场
Maryland Offers Support to Chinese Biopharmaceuticals in Entering US Market

王艳波 Vikki WANG
马里兰州商务厅
Maryland Department of Commerce
关于艾贝泰
艾贝泰生物科技有限公司(Applitech Biological Technology Co., Ltd.)作为一家集设计、研发、生产、销售和服务于一体的高新技术企业,致力于为生物制药领域提供专业的生产及分析设备、一次性耗材和整体解决方案。从成立至今,我们始终以客户为中心,将“质量为本,服务为先”作为经营方针,立足于生物工艺的优化、放大和生产,不断完善生物制药领域的产品线,为用户提供全方位生物工艺的专业解决方案,助力用户在生物制药领域不断取得新的突破。
