Characterization of a PolyesterResin/Catalyst System by TGA, DSC, and DMA
Thermal Analysis encompasses a family oftechniques that, when used together to characterize material properties, will yieldimportant information regarding how materials will perform under a wide rangeof operating temperatures. DSC measures the temperatures and heat flowsassociated with transitions in materials as a function of temperature or time ina controlled atmosphere. This technique provides quantitative and qualitativeinformation about physical and chemical changes that involve endothermic orexothermic processes, or changes in heat capacity. Thermogravimetric Analysis(TGA) measures the amount and rate of change in sample weight as a function oftemperature or time. DMA measures the modulus (stiffness) and damping (energy dissipation)properties of materials as the materials are deformed under a periodic stress.
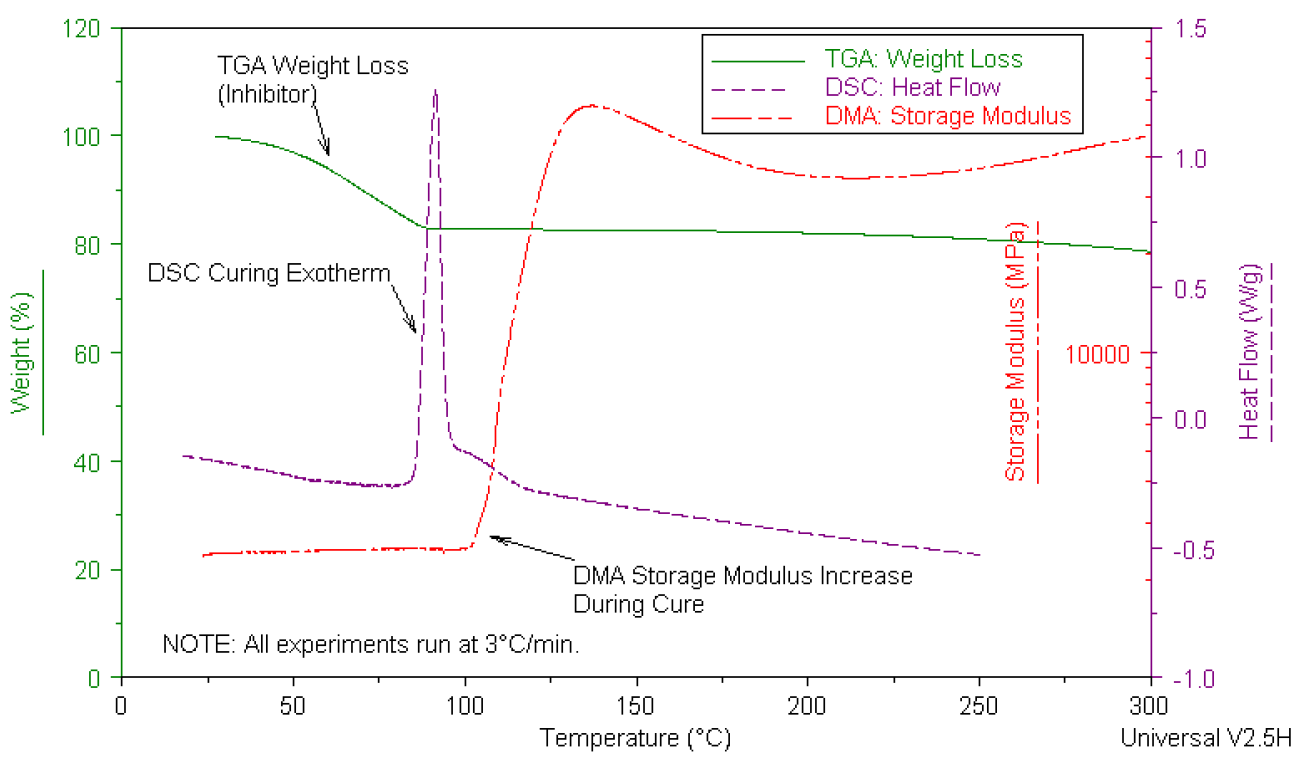
The above plot demonstrates how DSC, TGA,and DMA were used to characterize the curing behavior of a polyesterresin/t-butyl perbenzoate catalyst system. For the system to cure, theinhibitor must be removed from the sample. As the sample is heated, TGA detectsthe weight loss associated with the loss of inhibitor between room temperatureand 75¡C. Immediately after the loss of inhibitor, DSC detects the exothermassociated with the cross-linking/cure reaction. When a material cross-linksthere is a significant change in the mechanical properties of the sample. DMA easilydetects the rapid increase in modulus at the later stages of cure. Because DMAmeasures the physical and mechanical changes in a material, this technique is inherentlymore sensitive to the final stages of cure compared to DSC. This example showshow data from multiple thermal analysis techniques can be correlated to betterunderstand the thermal properties of a sample.

